Glp Sop Template
Simply go through the available sops and update with your facility information adapting them to best suit your needs.
Glp sop template. Select the sops you would like to view. Process cleaning and methodology validation regulatory auditing created for small and medium size pharmaceutical manufacturing environments. Glp sops for equipment calibration and maintenance. However when youre starting out with your first sops it can be difficult to know where to begin.
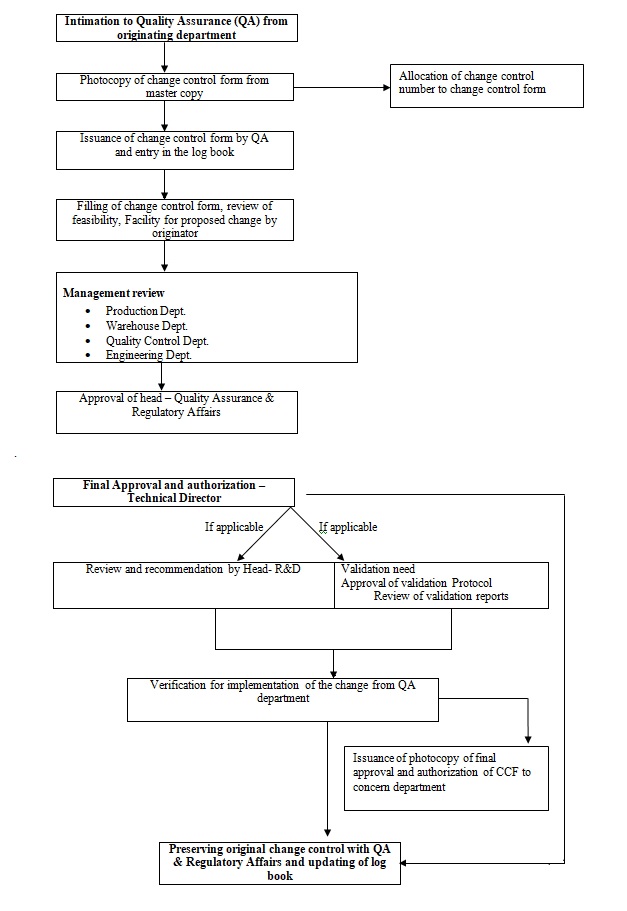
Sop for good laboratory practice glp standard operating procedure of good laboratory practice to avoid the errors inherent during performance of any analysis. Sopema0040 evaluation of conflicts of interests of experts for involvement in ema activities. Step by step pre written standard operating procedures forms templates and manuals in the area of gmp good manufacturing practice glp production operations quality assurance management quality control microbiology laboratory. Soppd10202 standard operating procedure good laboratory practice valid for.
A descriptive title and statement of purpose. 2 years from approval page 2 of 11 1. Thats why weve pulled together a complete package of sop templates for sqf ed8 modules 2 and 11 for you to work from. Scope this standard operating procedure sop applies to the staff and students of the pharmacy department to follow the good laboratory practice glp.
The true value of the sop on sops is often underestimated. Refer to texas am protocol sop tamu 01 006 glp protocols must contain the following information at a minimum. Sop templates and sop on sops irina colligon1 and michelle rosa2 1downingtown pa usa 2bethlehem pa usa summary document templates are valuable tools and standard operating procedure sop templates are no exception. Pharmaceutical guidanace april 9 2016 qa qc quality control sop comments off on sop on good laboratory practices glp 159 views related articles deviation handling and quality risk management as per who.
Standard operating procedures sops are developed by epa as guidance for inspectors done under the good laboratory practices glps program as set forth in the federal insecticide rodenticide and fungicide act and the toxic substances control act. Directive 200410ec on the principles of glp. Directive 20049ec on the inspection and verification of glp. The sops only are directive in nature for epa.