Fda Export Certificate
In addition to issuing export certificates for licensed approved or cleared products the fda will also issue export certificates for unapproved products that meet the requirements of sections.
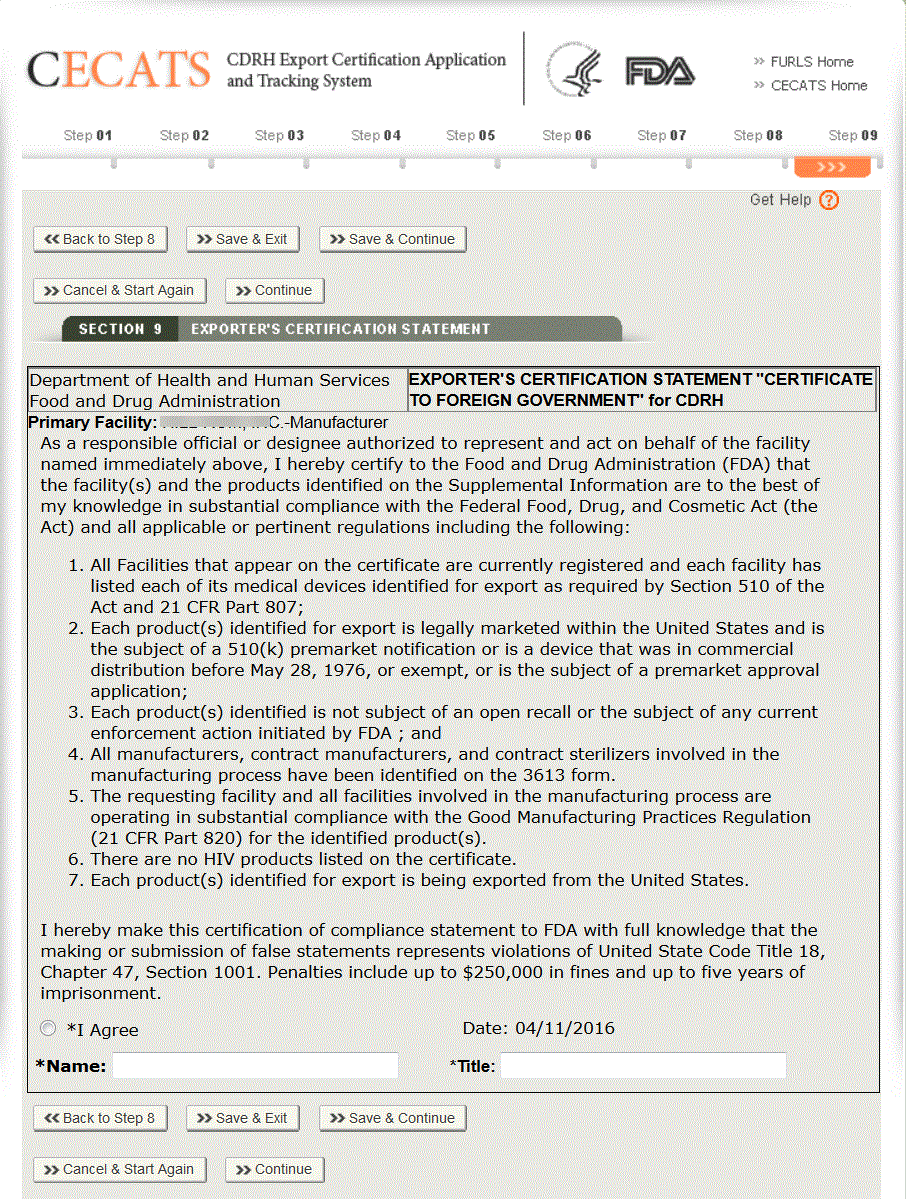
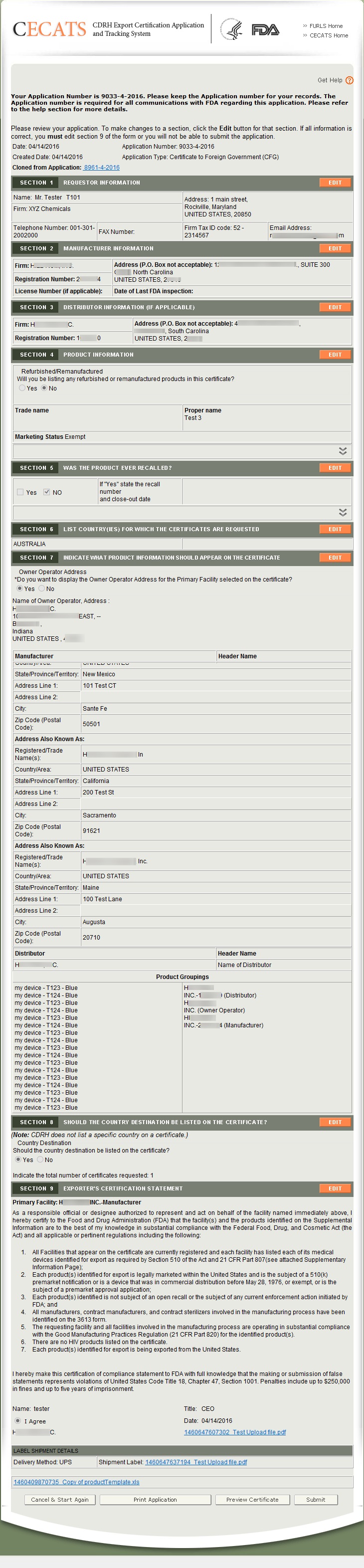
Fda export certificate. This certificate states among other things that a product or products. The certificate to a foreign government is available for conventional foods food additives food contact substances and infant formula that meet the applicable requirements of the fdc act for marketing in the united states. Firms exporting products from the united states are often asked by foreign customers or foreign governments to supply a certification relating to products subject to the federal food drug. Industry may request most types of fda issued export certificates for food products through the cfsan export certification application and tracking system cfsan ecats.
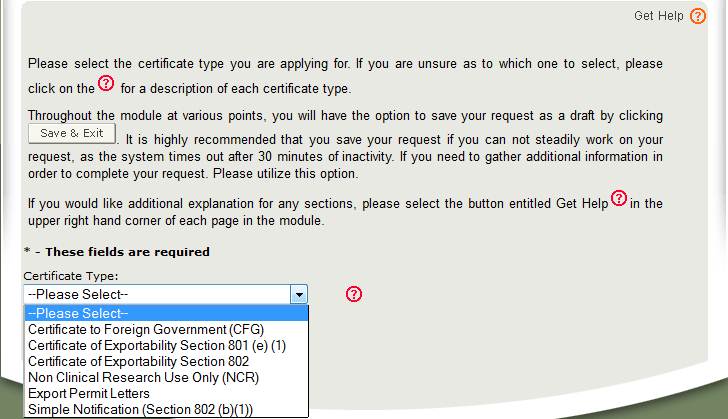
Cfsan ecats has different modules for different types of export certificates. This guidance document is intended to provide a general description of fda export certificates to industry and foreign governments. Foreign governments may seek official assurance that products exported to their countries can be lawfully marketed in the united states or that the products meet specific us.